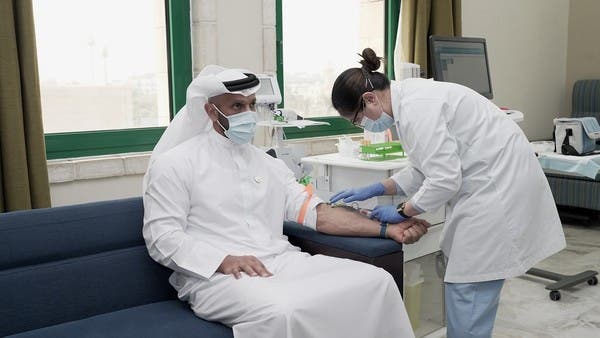
Just yesterday, the UAE announced the approval of coronavirus vaccines for front-line workers. Minister of Health and Prevention, Abdul Rahman Al Owais, said in a press briefing, “The vaccine’s safety has been reviewed and results show it is safe for use,” explaining that throughout the final stages of the third phase clinical trials of the vaccine, the results show that it’s effective and actually has a strong response, generating antibodies to the virus.
The vaccine was developed by the China-based company, Sinopharm, where phases one and two of the trials were performed successfully. The UAE is involved in an agreement with technology company Group 42 and was chosen for phase three.
Chairperson of the National Clinical Committee for Coronavirus and the principal investigator of the third phase of clinical trials of the inactive vaccine to combat COVID-19, Dr. Nawal Al-Kaabi, added during the briefing that over a period of six weeks, 31,000 people from 125 different countries took part in the vaccine clinical trials. “The preliminary results are encouraging, however, studies will continue. Side effects are simple and come as expected just like any other vaccine. No dangerous side effects or symptoms have been reported,” said Al-Kaabi. The vaccine was tested on 1,000 volunteers who suffer from chronic diseases and during the trial, they only felt minor symptoms such as a sore throat.
The clinic was set up at Abu Dhabi National Exhibition Centre to manage the trial and another was later established in Sharjah. A large number of medical staff came forward as well to offer their support to the project, Dr. Al Kaabi said the results of the clinical trials were “very positive”.